Coronavirus case in Africa
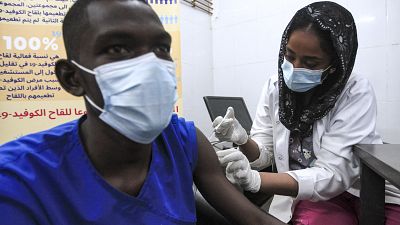
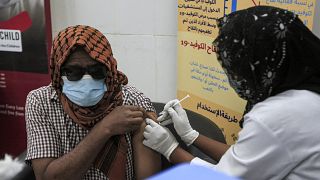
As several European countries suspend their rollout of the Oxford-AstraZeneca coronavirus vaccine, many African countries are continuing with it.
Many nations have suspended the Oxford-AstraZeneca vaccine as there are concerns that it may cause blood clots.
On Tuesday, the Africa Centres for Disease Control (Africa CDC) said it is reviewing its guidance of the vaccine.
Speaking with Bloomberg TV, Africa CDC Director John Nkengasong said: “The AstraZeneca vaccine was seen to be safe and efficacious and we would need to review the data. We should guide the response with strong science and evidence.”
He said the Africa CDC was convening an emergency meeting on Tuesday afternoon to look at the data and provide guidance.
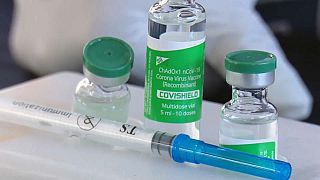
Many countries in Africa are relying on the AstraZeneca vaccine as it is cheaper and easier to store.
Under the Covax initiative, around 14,5 million doses have been delivered to African countries.
The Democratic Republic of Congo is the only African nation to postpone the start of its AstraZeneca vaccination campaign over blood clot fear.
The DRC received 1.7 million doses of the AstraZeneca vaccine and was due to start its rollout on Monday.
Meanwhile, health authorities in Uganda and Nigeria said they believe the vaccine is safe.
The World Health Organization said there is no evidence of a link but is reviewing the situation.
The Anglo-Swedish pharmaceutical giant AstraZeneca insists the jab is safe and that "no evidence" exists of a higher risk of blood clots.










01:57
Senegal beach fitness club offers hope and healing through water aerobics
01:00
Malawi launches cholera vaccine rollout
01:28
United States officially exits the World Health Organization
01:05
Uganda to receive $1.7 billion of US funding under new health deal
02:09
Toxic smoke chokes Conakry community as residents plead for government action
01:00
Tehran's severe pollution forces school closures and limits traffic